
Health
UN chief receives COVID-19 vaccine in New YorkBy Deepak Arora
In a Tweet, Guterres expressed his gratitude and good fortune at receiving the jab, and urged the international community to ensure that vaccines becomes available to everyone, on an equitable basis. “With this pandemic, none of us are safe until all of us are safe,” he wrote. 71-year-old Guterres was eligible to receive the vaccine on the basis of his age: New York residents over the age of 65 are included in the current phase of vaccinations in the city, which also includes school workers, first responders, public transit workers and grocery workers. In December, Guterres declared that he would happily receive the vaccine in public, and said that, for him, vaccination is a moral obligation: “Each one of us provides a service to the whole community”, he said, “because there is no longer a risk of spreading the disease.” Journalists and camera crews were invited to observe the UN chief receive his shot, which took place at a time when many countries are seeing a significant proportion of their citizens expressing “vaccine hesitancy”. UN regional offices have noted a significant level of mistrust and, in some countries, including Japan and several European nations, around half the population are reportedly unsure about getting a COVID-19 vaccine at this stage. Commenting on the UN chief’s vaccination appointment on behalf of the Mayor’s Office, Penny Abeywardena, Commissioner for International Affairs, said that she was heartened that the Secretary-General had secured his appointment online, and received the vaccine in a New York City public school, in the same manner as many other city residents. “This will go a long way in building trust in our communities that the vaccine is safe for all”, she said. India to launch new Covovax vaccine by JuneMUMBAI, Jan 30: A day after Serum Institute of India (SII) sought the Drugs Controller General of India’s approval to conduct a small domestic trial of the Novavax coronavirus vaccine, its Chief Executive Officer Adar Poonawalla on Saturday the company is hopeful of launching the vaccine by June this year. The vaccine will be launched under the local brand Covovax. Poonawalla said that SII's partnership with Novavax, a vaccine undergoing trials for effectiveness against the novel coronavirus, has published 'excellent efficacy results'. “Our partnership for a COVID-19 vaccine with @Novavax has also published excellent efficacy results. We have also applied to start trials in India. Hope to launch #COVOVAX by June 2021!” he said in a tweet. Pune-based SII, the world's largest vaccine manufacturer, and the Indian Council of Medical Research have a tie-up with the company and bridging studies will be done once the regulators give the approval. SII's application with the DCGI is being examined. A bridging trial is a supplementary trial performed in a new region or country to get more clinical data on efficacy, safety and dose regimen. Earlier, Dr Umesh Shaligram, executive director of SII, said that the company is keen on starting the bridging trials in the country and that SII would be in a position to start the trials by February once the approvals come through. “Data of protection against the new variants is promising. The good thing is that the new strain in South Africa emerged as the trials of the vaccine were going on in the country,” he said. In September 2020, Novavax had announced its deal with Serum Institute to produce 2 billion doses of the Covid-19 vaccines. In India, the vaccine will be called Covovax, developed by Novavax and upscaled by the SII. Johnson & Johnson 1-dose shot prevents COVID-19WASHINGTON, Jan 29: Johnson & Johnson’s long-awaited vaccine appears to protect against COVID-19 with just one shot – not as strong as some two-shot rivals but still potentially helpful for a world in dire need of more doses. J&J said Friday that in the U.S. and seven other countries, the single-shot vaccine was 66% effective overall at preventing moderate to severe illness, and much more protective — 85% — against the most serious symptoms. There was some geographic variation. The vaccine worked better in the U.S. — 72% effective against moderate to severe COVID-19 – compared to 57% in South Africa, where it was up against an easier-to-spread mutated virus. “Gambling on one dose was certainly worthwhile,” said Dr. Mathai Mammen, global research chief for J&J’s Janssen Pharmaceutical unit. With vaccinations off to a rocky start globally, experts had been counting on a one-dose vaccine that would stretch scarce supplies and avoid the logistics nightmare of getting people to return for boosters. The company said within a week, it will file an application for emergency use in the U.S., and then abroad. It expects to supply 100 million doses to the U.S. by June, and expects to have some ready to ship as soon as authorities give the green light. These are preliminary findings from a study of 44,000 volunteers that isn’t completed yet. Researchers tracked illnesses starting 28 days after vaccination – about the time when, if participants were getting a two-dose variety instead, they would have needed another shot. After day 28, no one who got vaccinated needed hospitalization or died regardless of whether they were exposed to “regular COVID or these particularly nasty variants,” Mammen said. When the vaccinated did become infected, they had a milder illness. Defeating the scourge that has killed more than 2 million people worldwide will require vaccinating billions, and the shots being rolled out in different countries so far all require two doses a few weeks apart for full protection. Early data is mixed on exactly how well all the different kinds work, but shots made by Pfizer and Moderna appear to be about 95% protective after the second dose. But amid shortages, some countries have advised delaying the second dose of certain vaccines with little data on how that would affect protection. All COVID-19 vaccines train the body to recognize the new coronavirus, usually by spotting the spikey protein that coats it. But they’re made in very different ways. J&J’s shot uses a cold virus like a Trojan horse to carry the spike gene into the body, where cells make harmless copies of the protein to prime the immune system in case the real virus comes along. Rival AstraZeneca makes a similar cold virus vaccine that requires two doses. Both the AstraZeneca and J&J vaccines can be stored in a refrigerator, making them easier to ship and to use in developing countries than the frozen kind made by Pfizer and Moderna. It’s not clear exactly how well the AstraZeneca version, being used in Britain and several other countries, works. Tests in Britain, South Africa and Brazil suggested two doses are about 70% effective although there are questions about how much protection older adults get. An ongoing U.S. study may provide more information. J&J said its vaccine works consistently in a broad range of people: A third of participants were over age 60, and more than 40% had other illnesses putting them at risk of severe COVID-19, including obesity, diabetes and HIV. J&J said the vaccine is safe, with reactions similar to other COVID-19 shots such as fever that occur when the immune system is revved up. While it released few details, the company said there were no serious allergic reactions. But occasionally other COVID-19 vaccines trigger such reactions, which can be reversed if promptly treated – and authorities have warned people to be on the lookout regardless of which type of vaccine is used. J&J had hedged its bets with a study of a two-dose version of its vaccine, which is still underway. Friday's interim results come on the heels of another vaccine in final testing. Novavax reported this week that its vaccine appears 89% effective in a U.K. study and that it also seems to work — though not as well — against new mutated versions of the virus circulating in Britain and South Africa. A larger study in the U.S. and Mexico is still enrolling volunteers. Pfizer-BioNTech Covid vaccine works against UK, South AfricaWASHINGTON, Jan 28: Apart from Pfizer, Moderna's coronavirus vaccine also appears to work against the new and more infectious variants of Covid-19 found in the UK and South Africa. Pfizer and BioNTech, makers of a coronavirus vaccine, on Thursday said that their product is effective against the Covid-19 variants that have emerged in the United Kingdom and South Africa. In a statement, the two companies said these preliminary findings "do not indicate the need for a new vaccine to address the emerging variants". They said they are "prepared to respond" if a new strain is shown to be able to evade the immunity of the vaccine, adding that they can produce updates to their jab if needed. The statement comes after US biotech firm Moderna this week announced that its coronavirus vaccine also appears to work against the new and more infectious variants of Covid-19 found in the UK and South Africa. Early laboratory tests suggested antibodies triggered by the Covid-19 vaccine can recognise and fight the new variants. However, more studies are needed to confirm this is true for people who have been vaccinated. Covaxin effective on UK virus strain of Covid-19 Meanwhile, an Indian Council of Medical Research (ICMR) study on India-made Covaxin shows a comparable neutralisation activity of the vaccinated individuals against the new UK-variant strain. The ICMR's National Institute of Virology scientist performed the plaque reduction neutralisation test using sera collected from the 26 recipients of Bharat Biotech's coronavirus vaccine (Covaxin) against UK mutant virus. "A comparable neutralization activity of the vaccinated individuals sera showed against UK-variant and the heterologous strain with similar efficiency dispel the uncertainty of possible neutralization escape," stated document appeared in bioRxiv in a pre-print version. "We successfully isolated and characterized the SARS-CoV-2 from UK returnees in India with all signature mutations of the UK-variant," noted the NIV-Pune scientist. "We present the neutralizing antibodies (Nab) titers to underline the efficacy of Covaxin vaccine candidate against SARS-CoV-2 UK-variant and one of the heterologous strains. Sera collected from 38 vaccine recipients, who received Covaxin vaccine-candidate in phase-II trial had equivalent NAb titers to homologous strain and two heterologous strains with the characteristic substitution of the UK-variant," said the authors. "The median ratio of 50 per cent neutralization of sera was found to be 0.8 and 0.9 when compared with earlier detected SARS CoV-2 strains against mutant UK strain," they said. "Our study evidently highlighted comparable neutralization activity of vaccinated individuals sera against variant as well as heterologous SARS-CoV-2 strains." "Importantly, sera from the vaccine recipients could neutralise the UK-variant strains discounting the uncertainty around potential escape. It was reassuring from the neutralizing antibodies data generated in our laboratory that the indigenous Covaxin following its rollout in the vaccination program, could be expected to work against the new UK-variant. It is unlikely that the mutation would be able to dampen the potential benefits of the vaccine in concern," it said. The new variants have been spreading fast in a number of nations. In India, the number of people who have tested positive for the new UK variant of Covid-19 has climbed to 153, Union Health Minister Harsh Vardhan said on Thursday. World On Verge Of Defeating Covid Pandemic, Says Harsh Vardhan At WHO MeetNEW DELHI, Jan 27: The world is on verge of defeating the coronavirus pandemic by adopting preemptive, proactive and collaborative strategies to combat the virus, said Union Health Minister Harsh Vardhan on Tuesday. "I thank member nations that despite the wide disparity in their epidemiological trends, we're on verge of defeating pandemic by adopting preemptive, proactive and collaborative strategies," said the Union Health Minister at 148th session of WHO Executive Board (virtual) meeting. Speaking about the year that was marred by the pandemic, he said, "2020 was the year of science when the best of humanity was shown through the gloom that descended due to the COVID-19 pandemic. The situation demanded setting up of major global collaboration so that scientists could share their expertise. For these governments, business and philanthropic organisations got together to start committing resources." On the efforts being undertaken globally to fight COVID-19, Harsh Vardhan added: "All member states are doing their utmost to overcome challenges and improve the accessibility, affordability and quality of health care services. But the job isn''t finished here. So we must redouble our commitment to putting an end to the pandemic." "In the present context, nowhere is safe until everywhere is safe. At the WHO, we have ensured that the low and middle-income countries have access to safe and effective COVID-19 tests, treatment and vaccine." Over the post-COVID world, he said, "The post-COVID world will be filled with challenges. To overcome them, WHO needs to be a trailblazer, empowered to work towards a new vision that allows it to realise its full potential and strengthen health care systems across member nations." Harsh Vardhan's statement comes as the worldwide tally of COVID-19 cases surpassed the 100 million mark on Wednesday, according to a tally by Johns Hopkins University. The United States continues to be the worst affected country with 25,407,414 cases. 447 reported adverse effects after Covid-19 vaccination, 3 hospitalised: Indian GovtNEW DELHI, Jan 17: More than 224,300 people have been vaccinated against the coronavirus disease so far, out of which only 447 reported adverse effects and only three had to be admitted to hospitals, the Union health ministry said on the second day of the nationwide vaccination drive on Sunday. The health ministry said 17,072 people received the vaccine on Day 2 of the vaccination drive in six states. “A total of 447 adverse events following immunisation (AEFI) reported so far, of which only three cases required hospitalisation,” said Manohar Agnani, a senior health ministry official. Agnani said that most of the adverse events following immunisation (AEFI), which were reported, were minor cases such as fever, headache and nausea. "Today being Sunday, only six states conducted vaccination drive and in 553 sessions a total of 17,072 beneficiaries were vaccinated," he said while addressing a press briefing. Andhra Pradesh, Arunachal Pradesh, Karnataka, Kerala, Manipur and Tamil Nadu carried out the vaccination drive on the second day, he added. The health ministry also lauded vaccinators for ensuring that India inoculated more people compared to other countries on the first day of its vaccination. It said India has the highest number of people vaccinated on the first day of the Covid-19 vaccination drive with close to 225,000 vaccinations. The Delhi government had said on Saturday that 52 people who had been administered the Covid-19 vaccine had reported adverse event post-inoculation. Out of these 52, one case was deemed serious by doctors. The other 51 beneficiaries were discharged after they recovered. Telangana also reported 11 cases of adverse events on the first day of the vaccination drive and West Bengal reported 14 such cases on the same day. India on Saturday launched the world’s largest vaccination campaign in a bid to curb the spread of coronavirus and aims to vaccinate 30 million frontline workers in the first phase of the programme. Microsoft Launches Digitial Vaccination Passport Plan With Global Healthcare OrganizationsNEW YORK, Jan 16: Microsoft is joining forces with tech companies and health organizations to begin development of a digital COVID-19 vaccination passport. The digital vaccination passport will be made available worldwide and could become a new standard for international travel in the post-pandemic world. The Vaccination Credential Initiative (VCI) is a coalition of leading tech companies and healthcare organizations seeking to enable transparent vaccination record passports. The idea is that once you have a COVID-19 vaccination, you can carry a digital verification of the vaccination. The digital vaccination passport will enable you to travel, return to work and school, and complete other "normal" tasks currently restricted due to the COVID-19 pandemic. VCI's vision is to empower individuals to obtain an encrypted digital copy of their immunization credentials to store in a digital wallet of their choice. Those without smartphones could receive paper printed with QR codes containing W3C verifiable credentials. Microsoft is just one of the companies joining the VCI. Other tech companies include Oracle and Salesforce, who are joined by health organizations, including The Commons Project, The Mayo Clinic, and Safe Health. Paul Meyer, CEO of The Commons Project Foundation, confirms that "The goal of the Vaccination Credential Initiative is to empower individuals with digital access to their vaccination records," using an open standard to protect privacy and begin to open the world back up. The VCI will use the open SMART Health Cards specification, which is being developed as a short term solution "to enable a consumer to receive COVID-19 Immunization or lab results and present these results to another party in a verifiable manner." The Vaccination Credential Initiative will work alongside the World Health Organization (WHO) to ensure the digital vaccination passport will eventually become available worldwide. The introduction and rollout of a global digital vaccination passport are likely to prompt understandable pushback. The privacy implications of such an initiative will come under scrutiny, with many arguing that they shouldn't have to disclose their vaccination to anyone, period. It is hard not to agree. However, the flip side is that some countries may begin restricting entry based upon COVID-19 vaccination status, especially if the pandemic doesn't show any sign of abating. Against a backdrop of increasing infection numbers and new COVID variants, countries may require travelers to present a valid digital vaccination passport before entry. As not everyone will have a COVID vaccination (for whatever reason, but we're not getting into that), it could place additional and potentially unnecessary restrictions on regular people. 23 die in Norway after receiving Pfizer COVID-19 vaccineOSLO, Jan 16: Twenty-three people died in Norway within days of receiving their first dose of the Pfizer COVID-19 vaccine with 13 of those deaths apparently related to the side effects of the shots, New York Post reported citing the health officials. All 13 were nursing home patients and at least 80 years old. Common reactions to the vaccine, including fever and nausea, "may have contributed to a fatal outcome in some frail patients," New York Post quoted Sigurd Hortemo, chief physician at the Norwegian Medicines Agency, as saying in a statement on Friday. While officials aren't expressing serious concern, they are adjusting their guidance on who should receive the vaccine. More than 30,000 people in Norway have received the first shot of the Pfizer or Moderna coronavirus vaccine since late last month. Agency's medical director Steinar Madsen has stated that the "agency is not alarmed by this." "It is quite clear that these vaccines have very little risk, with a small exception for the frailest patients. Doctors must now carefully consider who should be vaccinated. Those who are very frail and at the very end of life can be vaccinated after an individual assessment," he said. The agency reported Thursday that a total of 29 people had suffered side effects, including the 13 people who died. Twenty-one women and eight men experienced side effects, officials said. Besides those who died, nine had serious side effects -- including allergic reactions, strong discomfort and severe fever -- while seven had less serious ones, including severe pain at the injection site, New York Post reported. According to health officials around 400 people die each week in the nursing home population. A Pfizer rep said the company is "aware of reported deaths" following the administration of the vaccine in Norway and is working with the Norwegian Medicines Agency to gather all the relevant information. The total number of coronavirus cases reported in Norway is 58,202, while the death toll stands at 517, according to the Johns Hopkins University. Over 1.91 Lakh Vaccinated on Day 1 in IndiaNEW DELHI, Jan 16: The first COVID-19 vaccine shots in India were given on Saturday to nearly two lakh frontline healthcare and sanitary workers as Prime Minister Narendra Modi rolled out the world's largest inoculation drive against the pandemic that has caused 1,52,093 deaths and upended millions of lives in the country. As Modi asserted that the two vaccines being deployed will ensure a decisive victory for India against the coronavirus, the Union Health Ministry said no case of post-inoculation hospitalisation has been reported so far and the vaccination drive was successful. Sanitation workers were the first to get the jabs in Delhi and some states. Over 1.91 lakh beneficiaries were inoculated with COVID-19 jabs at 3,351 session sites across the country and no case of post-inoculation hospitalisation has been reported so far, the government said. The was successfully conducted on the first day, Additional Secretary in the Union health ministry Manohar Agnani said at a press briefing. "A total of 3,351 sessions were held wherein 1,91,181 beneficiaries were vaccinated, as per provisional reports," he said. A total of 16,755 personnel were involved in organising the vaccination sessions, he added. The 11 states and union territories where both Covishield and Covaxin were administered were Assam (65 sessions), Bihar (301), Delhi (81), Haryana (77), Karnataka (242), Maharashtra (285), Odisha (161), Rajasthan (167), Tamil Nadu (160), Telangana (14) and Uttar Pradesh (317). Prime Minister Narendra Modi launched the vaccination drive while reassuring the country that emergency use authorisation was given to two 'made in India' vaccines only after scientists were convinced of their safety and effectiveness, and urged people to beware of propaganda and rumours. More than one crore cases and 1.5 lakh fatalities later, India took its first steps out of the pandemic with shots of the Covishield and Covaxin vaccines being administered at medical centres. Sanitation worker Manish Kumar became the first recipient of vaccine at Delhi's All India Institute of Medical Sciences (AIIMS), according to the ministry. Of the first day beneficiaries, 2,897 were in Jharkhand, 12,637 in Karnataka, 7,206 in Kerala, 6,739 in Madhya Pradesh, 510 in Manipur, 509 in Meghalaya, 314 in Mizoram, 499 in Nagaland, 8,675 in Odisha, 206 in Puducherry, 1,200 in Punjab, 9,279 in Rajasthan, 120 in Sikkim, 2,728 in Tamil Nadu, 3,600 in Telangana, 266 in Tripura, 15,975 in Uttar Pradesh, 2,226 in Uttarakhand and 9,578 in West Bengal. Injecting confidence in people, several high-profile persons, including AIIMS director Randeep Guleria, NITI Aayog member VK Paul, who is also head of an empowered group on medical equipment and management plan to tackle the coronavirus outbreak, Serum Institute of India(SII) CEO Adar Poonawallah, West Bengal minister Nirmal Maji, BJP MP Mahesh Sharma, a doctor by profession, Apollo Hospitals Group Chairman Dr Prathap C Reddy and Manipal Hospitals Chairman Sudarshan Ballal received their first shot of the two-dose vaccine. Senior doctors received the first shot at many identified vaccination sites to dispel any apprehensions about the vaccine. Vardhan said the two vaccines -- Covaxin developed by Bharat Biotech and Covishield from the Oxford/AstraZeneca stable manufactured by the SII -- were a 'sanjivani', life infusing, in the fight against the pandemic. India has the second highest recorded cases of COVID-19 after the US. The caseload stood at 1,05,42,841 as on Saturday. Health workers who got their first shots of Covaxin at AIIMS were made to sign a consent form that promised compensation in case of a "severe adverse event" related to the vaccine. Covaxin has demonstrated the ability to produce antibodies against COVID-19 in phase one and phase two trials. "However, the clinical efficacy is yet to be established and it is still being studied in phase 3 clinical trial," the form read. Hence, it is important to appreciate that receiving the vaccine does not mean that other precautions related to COVID-19 need not be followed, it said. In his address, Prime Minister Modi reminded people that two doses of the vaccine are very important and asked them to continue with masks and social distancing even after receiving the jabs. The cost of vaccination of healthcare and frontline workers will be borne by the central government. Most hospitalised COVID-19 patients have at least one symptom 6 months after falling ill: StudyBEIJING, Jan 12: More than three quarters of Covid-19 patients hospitalised for treatment have at least one ongoing symptom six months after initially becoming unwell, according to a study published in The Lancet journal. The research looked at the long-term effects of the novel coronavirus infection in 1,733 patients first diagnosed in Wuhan, China between January and May followed to June and September. In the study, scientists, including those from Jin Yin-tan Hospital in China, interviewed the patients face-to-face using questionnaires to evaluate their symptoms and health-related quality of life. The discharged patients also underwent physical examinations, lab tests, and a six-minute walking test to gauge their endurance levels. Nearly 400 patients also underwent further tests, including an assessment of their lung function, and 94 patients whose blood antibody levels were recorded at the height of the infection received a follow-up test. According to the scientists, the most common symptom to persist was muscle weakness (63 per cent of cases), with patients also frequently experiencing sleep difficulties (26 per cent). They said anxiety or depression was reported among 23 per cent of patients. The study noted that hospitalised patients who were severely ill more often had impaired lung function and abnormalities detected in chest imaging -- which the scientists believe could indicate organ damage six months after symptom onset. Since very few follow-up studies have been conducted in recovered patients so far, the scientists said little is known about the long-term health effects of Covid-19. Those that have been conducted looked only at a small number of cases over a short follow-up period, they added. "Our analysis indicates that most patients continue to live with at least some of the effects of the virus after leaving hospital, and highlights a need for post-discharge care, particularly for those who experience severe infections," said study co-author Bin Cao, from National Center for Respiratory Medicine, China-Japan Friendship Hospital in China. "Our work also underscores the importance of conducting longer follow-up studies in larger populations in order to understand the full spectrum of effects that Covid-19 can have on people," Cao said. The scientists found that 76 per cent of patients reported at least one ongoing symptom during the follow up tests. Patients with more severe illness commonly had reduced lung function, with 56 per cent of those who required ventilation support experiencing reduced flow of oxygen from the lungs to the bloodstream. For patients who required supplemental oxygen therapy and those who did not require oxygen therapy, the researchers said the figures were 29 per cent and 22 per cent, respectively. According to the study, patients with more severe disease performed worse in the six-minute walking test. The scientists said 13 per cent of patients whose kidney function was normal while in hospital had reduced kidney function in follow-up. However, due to the way the data was analysed, the researchers said it was not possible to determine if symptoms reported during follow-up were persistent following the infection, worsened after recovery, or occurred post-discharge. The scientists believe further work is needed to compare differences in outcomes between inpatients and outpatients. ‘Give me one week’s time’: Bharat Biotech chief on questions about Covid-19 efficacyPUNE, Jan 4: Amid concerns regarding the efficacy data of Covaxin, developed by the Hyderabad-based Bharat Biotech, the company’s managing director Krishna Ella said that its vaccine is safe and the company is conducting trials in more than 12 countries apart from India. “We are not a company without experience in vaccines. We have tremendous experience in vaccines,” ANI quoted Ella as saying. “We are touching 123 countries. We are the only company that has got such extensive experience & extensive publication in review journals,” he also said. “We are not just conducting clinical trials in India. We have done clinical trials more than 12 countries including the UK. We are doing clinical trials in Pakistan, Nepal, Bangladesh & other countries. We are not just an Indian company, we are truly a global company,” Ella also said. On being asked about wether the vaccine will be effective on new strain of the virus, Ella said, “Give me one week’s time, I will give you confirm data.” The government recently approved emergency authorisation use of Oxford-AstraZeneca’s Covishield and also to indigenously developed Covaxin. Soon, data on Covaxin was sought by experts. Meanwhile, Indian Council of Medical Research (ICMR) Director General Dr Balram Bhargava had said on Sunday that Covaxin is based on an inactivated whole virus, having potential to target mutated coronavirus strains including the UK variant, and it was a major reason for giving it a conditional nod. Speaking on the controversy around the vaccine, Ella said, “Now that vaccine is being politicised, I want to state very clearly that none of my family members are associated with any political party.” “Approval of Covaxin for emergency use is a giant leap for innovation and novel product development in India. It is a proud moment for the nation and a great milestone in India’s scientific capability, a kickstart to the innovation ecosystem in India,” Ella also said. Bharat Biotech Covaxin has better efficacy: ICMR chiefNEW DELHI, Jan 3: The director general of the Indian Council of Medical Research Dr Balram Bhargava on Sunday said that the Bharat Biotech vaccine may have some advantages over other vaccines on the new mutant strain first detected in the UK, which has already infected 29 patients in India. His observations came amid politicians from several opposition parties raising doubts over the efficacy of Covaxin, which got approval for restricted emergency use in India. ICMR has collaborated with Bharat Biotech to develop this vaccine. Pune’s National Institute of Virology is another partner of this indigenous vaccine project. The NIV has also successfully isolated and cultured the UK-variant of the virus, first in the world, as ICMR said on Saturday. “NIV scientists have successfully isolated the new virus strain and this will be tested against different vaccines. We hope potentially Bharat Biotech vaccine will have some advantages over other vaccines on this new strain,,” Dr Bhargava said. Bharat Biotech’s Covaxin can be used as backup, says AIIMS Director Dr Randeep Guleria The third phase trial of the Bharat Biotech vaccine is going on. There is no data on its efficacy percentage so far, which is why the vaccine is facing so many questions. But the apex drug controller body has said that the vaccine has been found effective in its non-human and human trials so far. Though it has been approved for emergency use, its trial will continue. But what makes experts think that Covaxin might be more effective than Oxford’s Covishield to combat the new strain? Covaxin is a whole virion inactivated Covid-19 vaccine while Oxford vaccine works differently — it produces surface spike protein priming the immune system to attack the SARS-CoV-2 virus if it later infects the body. AIIMS chief Dr Randeep Guleria too has said that Bharat Biotech vaccine will be a back-up or for emergency use where the effectiveness of the Oxford vaccine is not certain. “We don’t know for how long a vaccine is going to be effective, we don’t know how much of the population we will have to vaccinate to break the virus transmission. What we know is that we have been able to control the pandemic in the country by following Covid appropriate behaviour,” said Bhargava. India Approves Two VaccinesNEW DELHI, Jan 3: In a short, eight-minute statement at 11 am on January 3, the Drug Controller General of India (DCGI) Dr V.G. Somani announced that his office had authorised two COVID-19 vaccine candidates for restricted use in the country. With the statement, India officially stood at the cusp of one of the world’s largest vaccination drives. But while the moment is suffused with hope, it will pay to remember at all times what exactly will happen from today: not a well-understood or well-planned event, and still hinged – for all the celebration of the science – on good luck. Within hours of the UK approving AstraZeneca’s COVID-19 vaccine candidate, the company began rolling out thousands of doses for distribution among the country’s high-priority groups. This kind of speed would have been possible only if AstraZeneca had assumed the candidate would be approved and had amassed enough doses for distribution at the country’s doorstep. But while this attitude could seem presumptuous and off-putting at other times, it’s apparently necessary during an ongoing pandemic – especially with a more contagious form of the virus showing up recently, and the AstraZeneca candidate being the cheaper option by far. At least, this is the excuse many people, including some experts, are advancing for rushing various aspects of vaccine production and distribution as much as possible. This is unfortunate. The pandemic is only part of the reason, and perhaps even in the minority, among all reasons that AstraZeneca is accelerating its candidate’s availability. A major one is that the Pfizer/BioNTech collaboration has taken a big lead in the US and the UK with its high-efficacy mRNA vaccine candidate, with Moderna a close second in the US market. All three candidates are two-shot vaccines, and the total population of the US and the UK is nearly 400 million. So given current manufacturing capacity, no single candidate will single-handedly be able to sate demands in both markets, especially since demand is also high. So all three candidates are certainly in contention. However, these companies have all been making their moves with at least one eye planted on the stock-markets. Stories of Pfizer’s windfall and the profits for its CEO when its candidate was approved for emergency use in the US are already part of pandemic lore. According to one estimate, the company is set to make $13 billion in profits in 2021 alone. So as such AstraZeneca is already late, and if it is to catch up, it must become available asap in the US and the UK, and try to press a first-mover advantage where it can. India is one such place. The Serum Institute of India has a deal with AstraZeneca to manufacture its candidate for sale in other markets, as well as to make and distribute doses for sale in India, under the name ‘Covishield’. On January 1, the subject expert committee of the Central Drugs Standard Control Organisation (CDSCO) recommended that the vaccine candidate be approved for restricted use. On January 3, DCGI Dr Somani approved the candidate, as well as Bharat Biotech’s counterpart, named Covaxin. The speed is awe-inspiring – but in India, it may also be a quirk of doing business. As it was reported, “American and European governments have promised vaccine-makers billions of dollars to offset potential losses” in exchange for the makers to start manufacturing even before candidates are approved. But at least as of August 2020, the Indian government hadn’t made any such promises, leaving only companies that could afford to write-off large losses with the ability to start manufacturing early. Serum Institute was one such company. Its CEO Adar Poonawalla told New York Times that it wanted to get a head-start because it could afford to, after investing upwards of Rs 3,300 crore. However, all of this is scant justification for how much we don’t know about Covishield, or about the other candidates for that matter – if only because we are at risk of fooling ourselves. In Covishield’s case at least, there is an unsettled dispute between one of its vaccine trial participants and Serum Institute. The former has sued the latter for Rs 5 crore, by way of compensation for a neurological side-effect that the participant has claimed arose as a result of being administered Covishield. Serum Institute subsequently threatened to counter-sue for Rs 100 crore, alleging defamation. Serum Institute’s response doesn’t inspire confidence in its version of events – more so since informed consent in the context of clinical trials is not as iron-clad an indemnification as some might think. AstraZeneca’s vaccine candidate is also knee-deep in trouble in the UK, where an advisory group to the government has expressed doubts about the company’s claim that the candidate could be more effective if the first of its two doses is halved in quantity. This claim arose from an accident during its phase 2 clinical trials, when a subgroup of participants received a full dose + half dose regimen by mistake, but which still reported 28 percentage points more efficacy. The company got in even more trouble after it became known that it may have cherry-picked data from its trials to share with governments for approval. Serum Institute only appears to be following suit. The company is conducting bridging trials in India, which can help find if a vaccine candidate tested elsewhere will elicit an immune response in the Indian population as well. Out of a pool of 1,600 participants, Serum Institute submitted data pertaining to only 100 volunteers to the CDSCO’s subject expert committee, The Hindu reported. Bear in mind that this is a vaccine to be distributed to over a billion people in India alone, and the committee has recommended it based on data from only a hundred of them. As for Bharat Biotech: the Covaxin vaccine candidate’s efficacy data isn’t in the public domain – nor are details from its phase 1 and 2 clinical trials and about how the company has estimated the figure to be around 60%. That the DCGI’s approval is entirely opaque only makes this more of a problem. That’s not all. The head of the UK advisory group, Munir Pirmohamed, recently said that when the group analysed the data AstraZeneca had submitted for its candidate’s approval, it found that the higher efficacy in the subgroup of participants that received the full-dose/half-dose regimen could in fact have been the result of a longer gap between the two doses, and not the altered dose volume. The UK has since approved a strategy to distribute the first dose to recipients as quickly as possible, since there is enough data to believe it provokes an immune response. However, we don’t know how the strength and duration of the response will vary in time, and whether the candidate will be just as efficacious if the second dose is delayed by more than a certain period. And yet again there is a caveat: the strategy to increase the gap between the two doses may not just be because the efficacy may be higher, but also because AstraZeneca may be having issues with supply! Isn’t this a shoddy path to vaccine approval, full of serendipities and experimental manoeuvres? As drug-discovery chemist Derek Lowe said, the pandemic, and the virus’s new mutation that allows it to spread faster through populations, may still be able to make a good case to support this kind of breakneck speed – but neither the UK government nor anyone else actually knows what they are doing. It’s a dice-roll (with a somewhat loaded die, but still) to be performed with millions of people, in the hopes that a vaccine candidate will work as intended. And AstraZeneca is not alone here. As Sandhya Srinivasan, consulting editor at the Indian Journal of Medical Ethics, wrote late last year, we don’t have vaccines – we have vaccine candidates. This is an important distinction because the candidates have been or are being tested in a way that doesn’t check if they’re good at preventing severe illness and if they are safe for the high-priority groups who will receive the first doses – especially the elderly. Both Srinivasan and S. Swaminathan, a retired scientist in New Delhi, have also expressed concerns about clinical trials overestimating vaccine efficacy by relaxing the threshold of effects. But the most pernicious defence of these practices has been to accuse anyone who asks uncomfortable questions of being “anti-vaxxers”. Anti-vaxx is short for ‘anti-vaccination’, a movement opposed to using vaccines to eliminate diseases because its members believe vaccines cause diseases instead. Anti-vaxxers are typically supporters of far-right ideologies, but those heaping the accusation on demands for accountability are assuming a similarly extreme, and unfeasible, position. In India at least, the drug approval process might as well not happen, considering the extent to which it is shrouded in secrecy, even as corporate leaders use high-handed tactics and even arguments of nationalism to shutdown complaints of malpractice and wrongdoing. Piling on top of this is the newly emerging – but historically familiar – “vaccine apartheid”. As The Intercept reported on December 31, 2020, the Pfizer/BioNTech vaccine candidate was tested in clinical trials with almost 44,000 participants across “Argentina, South Africa, Brazil, Germany, and Turkey as well as the US”. However, Argentina, South Africa, Brazil and Turkey will have to be satisfied with Pfizer’s gratitude, because … they won’t be receiving enough of the vaccine to inoculate their populations, at least not anytime soon. Meanwhile, the US and Germany — along with Canada and the rest of the EU — have contracted for enough doses of various COVID-19 vaccines to inoculate their populations several times over. A similar story of discrimination played out vis-à-vis severe adverse events during the trials of AstraZeneca’s candidate. When such an event was reported in the UK trials, the company responded by temporarily halting the trials and launching an enquiry based on a set of predetermined, well-understood guidelines. News of the event also hit the headlines worldwide within a day or two. Once the enquiry concluded, the trials resumed without any glitches. In India, however, Serum Institute claims it followed a similar process, as required under Indian law, but which culminated with the participant filing a case against the company. In addition, the event had occurred in early October but came to light only in late November. Other participants of the same trial also said they were contemplating legal action because they hadn’t been informed of the event. In the richer west, vaccine-makers’ attitude seems to be “what’s there to hide”. But in India, and other similar ‘developing countries’, it’s “what’s there to see”. This is why stakeholders – which is practically everyone – must ask more questions and seek more clarity about how vaccine candidates are being approved and sold in India. ‘Cancelling’ those who disagree would be tantamount to ‘cancelling’ ourselves. In a first, India successfully isolates, cultures UK-variant of Sars-CoV-2: ICMRNEW DELHI, Jan 2: In a first, India has successfully isolated and cultured the UK-variant of Sars-CoV-2, the virus that causes the coronavirus disease (Covid-19), the Indian Council of Medical Research (ICMR) said in a tweet on Saturday. “UK-variant of the virus, with all signature changes, is now successfully isolated and cultured at the National Institute of Virology (NIV) from the clinical specimens collected from UK-returnees,” the country’s apex medical body wrote on Twitter. It said that Sars-CoV-2, the virus causing Covid-19, was being tracked through the countrywide network of ICMR-laboratories since early days of the epidemic in India. No country has yet reported successful isolation and culture of the UK variant, according to ICMR. Vero cell lines were used by the scientists of ICMR-NIV to culture the UK-variant of the virus, it added. India has reported 29 cases of the new coronavirus variant, that is spreading rapidly around Britain and other countries, within five days of tracing its first such infection. The new United Kingdom variant genome of Sars-CoV-2 – B.1.1.7, which is much more infectious, has prompted comprehensive contact tracing for co-travellers, family members and others of those who have travelled to the UK in the last 38 days. From November 25 to December 23, 2020, midnight, about 33,000 passengers disembarked at various Indian airports from the UK. All these passengers are being tracked and subjected by states to RT-PCR tests to detect Covid-19. Apart from India, the presence of the new strain of the mutant virus has already been reported by Denmark, Netherlands, Australia, Italy, Sweden, France, Spain, Switzerland, Germany, Canada, Japan, Lebanon and Singapore. As a precautionary measure, the aviation ministry has restricted the operations of flights between India and the UK. Flights from India to the UK can start on January 6, while operations from the UK to India will begin on January 8. The schedule is valid till January 23, civil aviation minister Hardeep Singh Puri clarified on Saturday. The ministry has also issued a set of guidelines for travelling between the two nations. All passengers from the UK will have to submit self-declaration form on the online portal of Delhi Airport(www.newdelhiairport.in) at least 72 hours before the scheduled travel. They need to carry their negative report of the RT-PCR test, which should have been conducted within 72 hours prior to undertaking the journey. Passengers will have to undergo RT-PCR tests again upon arrival at their own cost. If tested positive upon arrival, passengers will have to be quarantined accordingly, the government has said. Bharat Biotech's Vaccine Cleared By Panel, Regulator's Approval AwaitedNEW DELHI, Jan 2: Covaxin, the coronavirus vaccine from Hyderabad-based Bharat Biotech, has been recommended for "restricted use in emergency situation in public interest" by a government-appointed panel, which submitted its findings to the Drugs Controller General of India on Saturday evening. The DCGI will take the final call on approving of the vaccine. The national regulator is scheduled to address the media at 11 am Sunday. The recommendation comes despite the lack of efficacy data at this time. Covaxin has completed only two of three trial phases; the third - which tests for efficacy and which the company has called "the largest... ever conducted in India" - began in November. Vaccine efficacy data is the result of combined analysis of all three phases. However, Dr Savita Verma, a pharmacology professor from Haryana's PGIMS who is part of the team working on the vaccine, said that "good efficacy was shown" in the earlier trial phases. "We have very robust Phase I and II results, in which good efficacy was shown. We are at present carrying out Phase III trials... have to recruit around 25,800 participants. As of now we have approximately 22,000 across India... we expect interim results by March," Dr Verma said. Dr Verma also said that around 10 million doses of Covaxin are ready at this point. Interim findings of Phase I trials showed Covaxin induces an immune response and registers no serious side effects. Phase II trial data showed "tolerable safety outcomes" and suggested antibodies may persist for six to 12 months. Restricted use approval is normally only granted if there is sufficient evidence to suggest the drug is both safe and effective. |
|
|||
Aviation
| Business | Defence
| Foreign Affairs | Communication
| Health | India |
United Nations
India-US | India-France
| Entertainment | Sports
| Photo Gallery | Tourism
| Advertise with Us | Contact
Us
© Noyanika International, 2003-2009. All rights reserved.
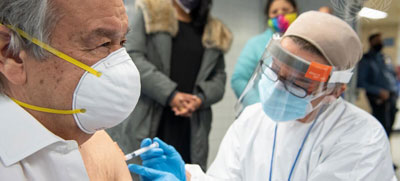 NEW YORK, Jan 29: The UN Secretary-General, António Guterres, received his first dose of the COVID-19 vaccine on Thursday, at Adlai E. Stevenson High School in The Bronx, a few miles uptown from UN Headquarters in New York.
NEW YORK, Jan 29: The UN Secretary-General, António Guterres, received his first dose of the COVID-19 vaccine on Thursday, at Adlai E. Stevenson High School in The Bronx, a few miles uptown from UN Headquarters in New York.
